Clean Booth inspection before putting into production is a mandatory step to evaluate the performance, cleanliness and safety of the clean booth. The article “Clean Booth Inspection Checklist before putting into production” helps businesses understand the items that need to be checked to ensure the system meets ISO 14644 and GMP standards.
1. Purpose of Clean Booth Validation
Before official operation, validating the Clean Booth is a mandatory step to comprehensively assess its performance, cleanliness, and safety in a real production environment. The main objectives include:
- Ensure Clean Booth meets design standards: All technical specifications, materials, and equipment (such as FFU, HEPA Box, lighting...) must comply with the approved design drawings and meet the cleanliness level according to ISO 14644-1 or GMP standards.
- Evaluate real-world operation capability: Many Clean Booths meet design specifications on paper but fail to maintain ideal conditions during actual use. Validation helps detect issues such as airflow leakage, unstable cleanliness, or malfunctioning interlock doors.

- Prevent production risks and cross-contamination: Early validation helps companies avoid product quality issues, microbial contamination, or production shutdowns due to non-compliant environments-critical in pharmaceutical, food, electronics, and cosmetics industries.
See more: Latest price list of Clean booth used in food factory
2. When to Validate a Clean Booth
Clean Booth validation should not be a one-time activity. It must be conducted at various stages throughout its lifecycle-from installation to operation and post-maintenance. Each validation point serves a specific purpose:
|
Time |
Validation Objective |
|
After installation is completed |
- Assess construction and equipment installation quality - Check system integrity and airtightness |
|
Before official operation |
- Ensure environmental conditions (pressure, particle count, airflow…) meet ISO 14644 or GMP standards - Confirm the system can maintain stable cleanliness during production |
|
After system maintenance or upgrade |
- Evaluate the impact of changes (HEPA replacement, FFU upgrades, interlock repair…) on clean zone performance - Ensure the Clean Booth continues to perform effectively after technical interventions |
Choosing the right time for validation helps businesses prevent risks, ensure compliance, and avoid costly re-validations.
3. Clean Booth Validation Checklist
A compliant Clean Booth requires not just high-quality equipment, but also thorough validation of its design, systems, and environmental conditions. Below are the essential validation areas:
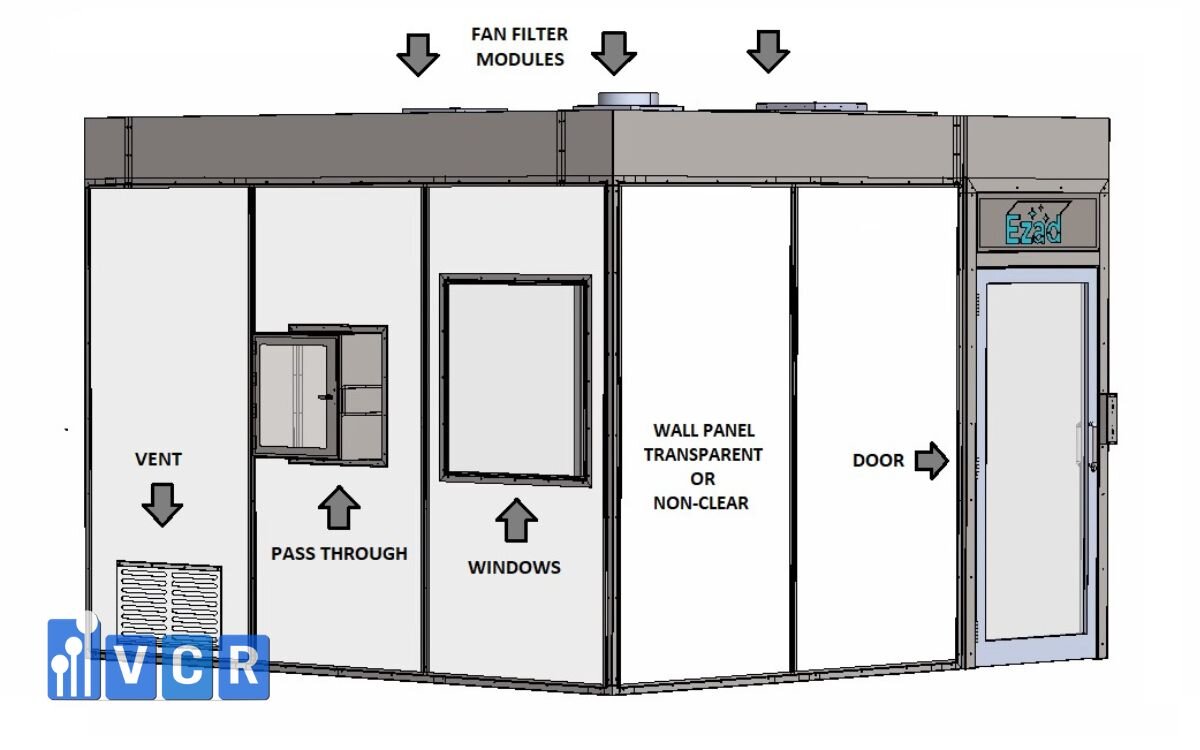
A. Design and Installation Check
Objective: Ensure the Clean Booth is built to design and material specifications.
- Actual dimensions: Match with approved design drawings
- Finishing materials: Inspect wall panels, ceilings, and anti-dust flooring for compliance
- Doors and viewing windows: Correct orientation, no warping
- Airtightness: Check for gaps, door seals, and leakage
B. Technical Equipment Check
Objective: Confirm the core equipment operates correctly and consistently.
- FFU (Fan Filter Unit): Measure airflow rate and uniformity
- Lighting: Ensure illuminance meets 300-500 lux (depending on ISO class)
- Sensors and door interlocks: Functional with proper response time
- HEPA Box: Installed in the correct airflow direction, no leaks at joints or frames

C. Environmental Conditions Validation
|
Validation Item |
Reference Standard |
|
Airborne particle count |
ISO 14644-1 |
|
Pressure differential between zones |
GMP EU Annex 1 |
|
Temperature - Humidity |
As per product requirements (typically 20-24°C, 40-60%) |
|
Air velocity (unidirectional flow) |
ISO 14644-3 |
All measurements should be conducted using regularly calibrated instruments with traceable certificates.
D. Documentation Required Before Validation
To ensure a smooth and transparent validation process, the following documents must be prepared:
- Design drawings and equipment layout
- Handover documents (with contractor sign-off)
- Technical datasheets (FFU, HEPA, Interlock, sensors…)
- Validation/qualification plan (IQ/OQ), if in a GMP-compliant facility
By strictly following this checklist, the Clean Booth will not only meet initial compliance but also ensure long-term stable operation-ready to serve high-standard production environments like pharmaceuticals, food, electronics, and cosmetics.
See more: Clean Booth or Fixed Clean Room: Which Solution is More Flexible?
4. Important Notes When Validating a Clean Booth
Although the Clean Booth validation process may vary depending on the factory scale and applied standards (GMP, ISO, HACCP…), there are several essential principles that must be followed to ensure legal compliance and effectiveness:
Prioritize validation by accredited organizations
Only select validation providers that are officially recognized (with ISO 17025 certification or equivalent).
They must use regularly calibrated instruments with traceable results.
Their technical team should be familiar with GMP/ISO requirements in industries such as pharmaceuticals, food, and electronics.
For GMP-compliant factories, this is a prerequisite for successful audits.
Validation reports must follow IQ/OQ (or FAT/SAT)
- IQ (Installation Qualification): Verifies the Clean Booth is installed correctly per design.
- OQ (Operational Qualification): Assesses operational performance under actual conditions.
For non-GMP factories, simplified formats such as FAT (Factory Acceptance Test) or SAT (Site Acceptance Test) may be used.
Each validation report should include images, measurement results, comments, and signatures from relevant stakeholders.
If deviations are found, a CAPA plan must be created
Common deviations include airflow leakage, insufficient pressure, reverse airflow, or inadequate lighting.
A corrective and preventive action plan (CAPA) must clearly define: who is responsible, timeline, and corrective steps.
After correction, re-validation is required to confirm compliance.
Do not operate the Clean Booth without an official acceptance report
Operating a Clean Booth without proper validation is a serious violation-especially in GMP-regulated environments.
It may result in failed audits, product recalls, and serious damage to the company’s reputation.
Acceptance of the Clean Booth is not just a technical requirement-it is a quality assurance commitment for the entire production line.
5. Who Should Perform Clean Booth Validation?
Clean Booth validation is an interdisciplinary process requiring close collaboration between multiple parties: installation contractors, QA/QC teams, independent validation service providers, and project investors. Each has distinct roles and responsibilities, all contributing to ensuring the Clean Booth meets technical standards and is ready for operation.
|
Role |
Key Responsibilities |
|
Contractor |
- Install equipment and materials according to design - Support initial inspection process - Provide technical documentation and completion reports |
|
QA/QC team |
- Review completeness of validation documentation (IQ/OQ) - Supervise measurement procedures: airflow, particle count, pressure… - Evaluate risks and recommend CAPA actions if needed |
|
Validation provider |
- Conduct on-site measurements with calibrated instruments - Compare results with ISO/GMP standards - Prepare detailed and transparent reports |
|
Investor / Project Manager |
- Approve IQ/OQ or FAT/SAT reports - Decide on official Clean Booth acceptance - Authorize operation if all criteria are met |
See more: GMP Standard Clean Booth Supplier in Vietnam
6. Clean Booth Validation
1. If I already installed certified HEPA filters, do I still need to validate the Clean Booth?
Yes. Installing certified HEPA filters is only part of the Clean Booth system. Full validation also includes:
- Measuring actual airborne particle counts
- Assessing airflow speed, pressure differential, and airtightness
- Evaluating overall system performance
Only when all environmental conditions meet ISO 14644 or GMP standards should the Clean Booth be authorized for operation.

2. How long does it take to validate a Clean Booth?
Typically, validation takes 1-3 working days, depending on:
- The size and cleanroom classification
- Number of test points as per layout
- Applicable standards (ISO, GMP, HACCP…)
Proper validation helps avoid delays in future audits-cutting corners may lead to greater costs later.
3. Can we perform in-house validation without hiring a third party?
Yes, under these conditions:
- The factory has a trained technical team
- Fully equipped with certified instruments for measuring particle count, pressure, airflow, and lighting
- Adheres strictly to ISO or GMP (IQ/OQ) validation procedures
However, for GMP-compliant or audit-ready facilities, using an independent validation provider increases credibility and helps ensure a successful audit outcome.
7. Need Help Validating a Clean Booth to ISO/GMP Standards?
Are you preparing to validate your Clean Booth but unsure about the technical requirements? Not sure how to start with IQ/OQ documentation? Don’t let a minor mistake delay your entire production schedule.
VCR Cleanroom Equipment is ready to support you with:
- Free consultation and validation checklist for each industry: pharmaceuticals, food, cosmetics, electronics
- On-site measurement using ISO 17025 certified instruments
- Assistance in preparing full IQ/OQ reports for audit readiness
- Coordination with engineering, QA, and contractors to ensure smooth and accurate validation
Hotline: 090.123.9008
Email: [email protected]
Website: https://cleanbooth.vn/
Diep VCR


















