Clean Booth for cosmetic R&D is becoming a prominent trend in 2026 thanks to its ability to control cleanliness, prevent contamination and protect operators during formula testing. With a flexible design, easy to install and ISO 22716 standard, Clean Booth helps optimize the modern cosmetic research process.
- 1. The Increasing Importance of Clean Booths in Cosmetic R&D
- 2. Automation and IoT Integration: A Key Trend for 2026
- 3. Modular Design: Optimizing R&D Space and Budget
- 4. Antibacterial and Easy-to-Clean Materials on the Rise
- 5. Compliant with ISO 22716 & ESG Standards
- 6. Comparison: Traditional Clean Booth vs. 2026 Clean Booth
- 7. Recommended Clean Booth Configuration for Cosmetic R&D
- 8. Frequently Asked Questions
- 9. Need a Clean Booth for Your Cosmetic R&D Lab?
1. The Increasing Importance of Clean Booths in Cosmetic R&D
In the cosmetics industry, the research and development (R&D) stage is not just about testing formulas - it is where new products are born. It’s also the most sensitive phase in terms of contamination risks, formulation instability, and regulatory non-compliance. Clean Booths - also known as mobile cleanrooms - are becoming an increasingly preferred solution in laboratories due to three key advantages:
Optimizing the Formula Testing Process
When testing multiple formulations in an open lab environment, the risk of component changes due to dust and microorganisms is high. Clean Booths offer a strictly controlled environment (typically ISO Class 6-8), helping to:
- Ensure accuracy of ingredients in each formulation test
- Minimize contamination that could affect product performance and stability
- Reduce trial-and-error time and accelerate product development
Protecting Products and Operators
Cosmetic ingredients - especially preservatives and fragrances - can cause irritation with prolonged exposure. Meanwhile, serum, gel, or emulsion products are highly prone to contamination. Clean Booths help:
- Create a clean air barrier around the working zone
- Prevent cross-contamination between samples
- Protect operators from inhaling fine particles or volatile chemicals
Complying with ISO 22716 & GMP Standards
ISO 22716 (Good Manufacturing Practices for cosmetics) and GMP guidelines require controlled environments for experimental production. Clean Booths help R&D labs:
- Establish a compliant working zone without needing a full-scale cleanroom
- Easily separate zones for filling, weighing, and sample storage
- Increase approval rates during GMP audits or facility assessments
2. Automation and IoT Integration: A Key Trend for 2026
By 2026, Clean Booths will no longer be just physical clean spaces - they are transforming into smart, mini laboratories integrated with digital technology. Thanks to IoT and smart sensors, next-gen Clean Booths support cosmetic R&D with greater efficiency and traceability.
Integrated Particle and Microbial Sensors
Cosmetic samples - especially serums or creams - are highly sensitive to microbial and particulate contamination. Modern Clean Booths feature PM2.5/PM10 sensors or ISO 14644-compliant particle counters to:
- Continuously monitor airborne particle levels
- Alert when thresholds are exceeded (e.g., ISO 7: 352,000 particles/m³)
- Minimize formulation errors caused by environmental variation
Automatic Pressure Control and Alarms
To block contaminated air inflow, modern Clean Booths are pre-programmed for positive pressure control. These systems:
- Automatically adjust FFU fan speeds based on preset pressure differentials
- Trigger alerts for pressure fluctuations, clogged HEPA filters, or open doors
- Log environmental data for quality inspections and internal audits
Real-time Dashboard Integration
Sensor data is fed into a cloud-connected dashboard, viewable on computers or smartphones - enabling cosmetic R&D teams to:
- Monitor environmental conditions during formulation trials
- Retrieve historical test environment data
- Include reliable metrics in audit reports or standard registrations
See more: Clean Booth for the Pharmaceutical Sector: GMP Installation Checklist
3. Modular Design: Optimizing R&D Space and Budget
Unlike mass production facilities, cosmetic R&D spaces are often compact and frequently reconfigured depending on project needs. This is why modular Clean Booths are gaining popularity - offering flexible installation and cost-efficiency.
Suitable for Labs of All Sizes
Modern Clean Booths use aluminum profiles or stainless steel frames and can be:
- Installed in 1-2 days without modifying existing infrastructure
- Custom-sized to fit available space (e.g., 2×2m, 3×4m, etc.)
- Relocated or reassembled easily as lab layouts evolve

Scalable for R&D Growth
Each cosmetic development project follows a unique cycle - from ideation → formulation → sample testing → pilot production. Modular Clean Booths allow:
- Starting with basic setups (FFU + PVC curtain)
- Gradual upgrades: solid panels, touchscreens, interlocks, UV lighting
- Seamless scaling when expanding formulation capacity
Cost-efficient Entry Point
Rather than investing in permanent cleanrooms, cosmetic startups or mid-sized labs can:
- Use Clean Booths to create dedicated clean zones for key tasks (e.g., weighing, filling)
- Eliminate the need for a separate HVAC system
- Optimize limited budgets while still meeting ISO 7-8 clean conditions
4. Antibacterial and Easy-to-Clean Materials on the Rise
In cosmetic R&D labs, where sensitive tasks such as weighing ingredients, emulsifying, and trial filling take place, fast cleaning and contamination control are critical. The 2026 trend highlights a clear shift from conventional materials to advanced antibacterial and easy-to-clean surfaces in Clean Booth construction.
Prioritizing Stainless Steel 304/316L for Frame and Surfaces
- Stainless Steel 304 is widely used due to its durability, corrosion resistance, and cost-effectiveness
- Stainless Steel 316L, a premium option, is ideal for areas requiring high cleanliness (low pH, exposure to solvents)
- Smooth, non-porous surface: prevents dust accumulation and is easy to disinfect with alcohol or cleaning agents
Nano Silver-Coated Panels for Active Antibacterial Protection
- Composite panels can be coated with nano silver (Ag⁺) to:
- Inhibit bacterial growth on surfaces for 24-48 hours
- Reduce the need for constant disinfection with strong chemicals
- Ideal for shared spaces where cross-contamination risks are high
Tempered Glass - Clear Visibility and Easy Cleaning
- Instead of frosted plastic curtains or acrylic sheets, 2026 Clean Booths prioritize tempered glass panels, offering:
- Clear visibility for internal operations (suitable for product demo or training)
- No static attraction like acrylic, easily cleaned with a dry cloth or RO water
5. Compliant with ISO 22716 & ESG Standards
By 2026, Clean Booths serve more than just clean zones - they help cosmetic manufacturers meet both international quality standards and sustainability commitments (ESG). These dual advantages make Clean Booths a strategic investment for growth.
Meeting ISO 22716 - GMP Requirements for Cosmetics
ISO 22716, the global standard for Good Manufacturing Practices in cosmetics, demands strict environmental control during product trials. Clean Booths allow R&D labs to:
- Create controlled work zones without building full-scale cleanrooms
- Easily divide areas for weighing, blending, filling, and testing
- Prevent cross-contamination between test formulas
- Increase chances of passing GMP inspections at the R&D stage
Supporting ESG: Energy Efficiency & Eco-Friendly Materials
ESG (Environmental - Social - Governance) has become a mandatory standard across FMCG and cosmetic sectors. Next-gen Clean Booths integrate ESG elements such as:
- Energy-saving FFUs operating efficiently in ECO mode
- Reusable frame systems, generating less construction waste than fixed cleanrooms
- Recyclable composite panels that reduce environmental impact
- Low-consumption LED lighting that still meets ≥500 lux requirements
Summary: ISO 22716 is the "gateway" for product approval, while ESG is the "door opener" for long-term brand credibility. A modern Clean Booth empowers cosmetic R&D teams to achieve both - smartly and affordably.
See more: Latest price list of Clean Booth used in electronics factory
6. Comparison: Traditional Clean Booth vs. 2026 Clean Booth
With increasing R&D demands and the rise of sustainable manufacturing, Clean Booths are no longer just technical tools - they represent a lab’s quality and ESG capability. Here's a quick comparison:
|
Criteria |
Traditional Clean Booth |
2026 Clean Booth |
|
Filtration Level |
ISO Class 8 |
ISO Class 6 or better (depending on FFU + HEPA H14 setup) |
|
Smart Control |
Not available |
Equipped with particle sensors, pressure control, alerts |
|
Design |
Fixed, time-consuming to install, non-portable |
Modular, upgradable, and relocatable |
|
Materials |
Basic panels, hard to clean |
Stainless steel 316L, tempered glass, nano silver panels |
|
ESG Compliance |
Not addressed |
Yes: energy-efficient, recyclable materials, low emissions |
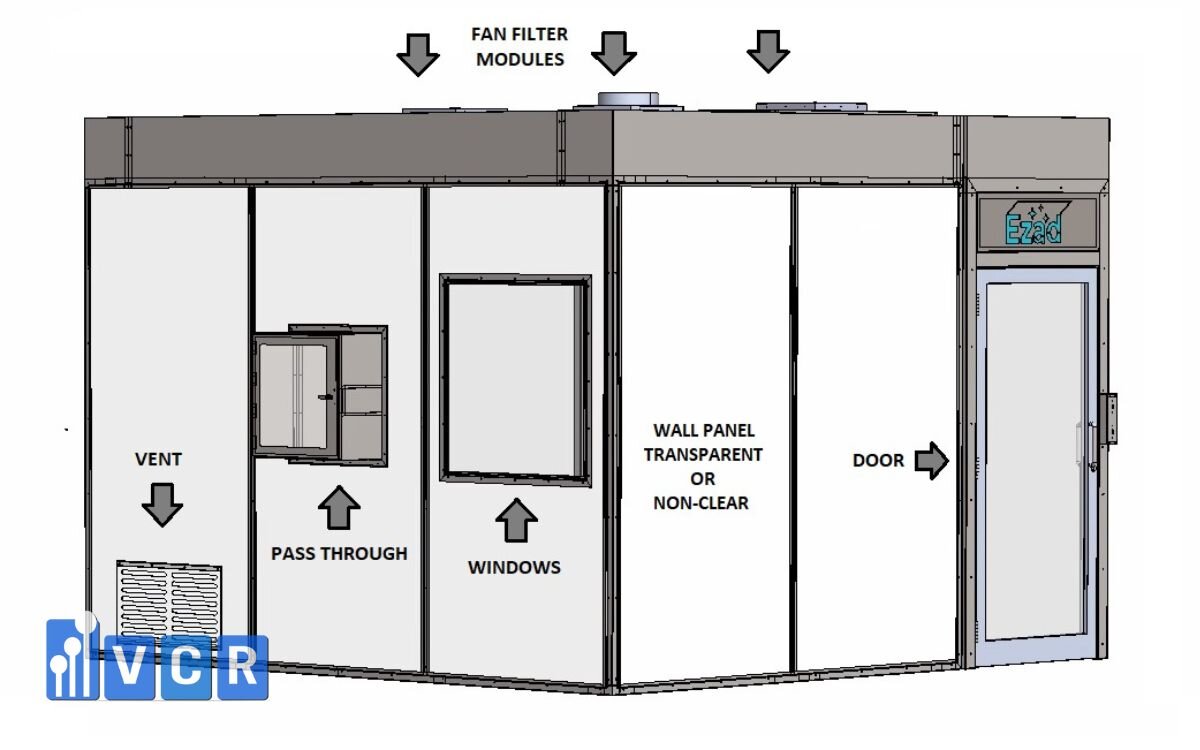
7. Recommended Clean Booth Configuration for Cosmetic R&D
To ensure operational efficiency, space optimization, and ISO 22716 compliance, here’s the ideal Clean Booth setup for modern cosmetic R&D labs:
Recommended Size: 6-12 m²
- Supports 1-3 staff working simultaneously
- Suitable zoning: weighing table - filling station - sample holding area
Filtration System:
- Ceiling-mounted FFUs with HEPA H14 filters
- Air velocity: 0.45 m/s ±20%
- Ensures ISO Class 7 or better

Lighting & Noise:
- Ceiling LED panels with ≥500 lux, neutral color temperature (4000-5000K)
- Operational noise: below 60 dB - suitable for focused work
Door System:
- Automatic sliding doors (single or double leaf)
- Integrated interlock system to prevent simultaneous access
- Optional features: safety sensors, observation glass, status indicator lights
8. Frequently Asked Questions
1. Should small-scale cosmetic R&D labs invest in a Clean Booth?
Yes. Even a compact Clean Booth (4-6 m²) can establish a controlled clean zone for sensitive procedures. It enables:
- Reduced risk of cross-contamination during formula trials
- Early compliance with ISO 22716 or GMP standards
- Easy expansion as R&D demands grow
2. What’s the difference between a Clean Booth and a fixed cleanroom?
|
Criteria |
Clean Booth |
Fixed Cleanroom |
|
Investment Cost |
Significantly lower |
High - requires structural renovation |
|
Setup Time |
1-3 days |
3-6 weeks or more |
|
Mobility |
Portable, easy to relocate |
Permanent, non-relocatable |
|
Best Use Case |
R&D, trials, localized clean areas |
Large-scale production |
Summary: Clean Booths are ideal for research environments with frequent process or layout changes.
3. How often should Clean Booths be maintained in cosmetic labs?
Recommended interval: Every 3-6 months, depending on:
- Usage frequency
- Type of product tested (liquid, emulsion, powder, etc.)
- Required cleanliness level of the area
Key maintenance checks include:
- HEPA H14 filter efficiency
- Pressure and particle sensor status (if applicable)
- Panel joints, air seals, and FFU vibration levels
9. Need a Clean Booth for Your Cosmetic R&D Lab?
Whether you're launching a small lab or preparing a certified R&D center, selecting the right Clean Booth configuration can save time, reduce costs, and prevent technical mistakes.
VCR’s engineering team has delivered hundreds of Clean Booth projects for the cosmetics sector - from local brands to international ODM manufacturers.
Free consultation available on:
- Custom configurations based on space, budget, and compliance level
- Required equipment for each stage of product development
- Modular upgrade paths and smart system integrations
Hotline: 090.123.9008
Email: [email protected]
Website: https://cleanbooth.vn/
Diep VCR


















